TEMPERATURE IN DEGREES C (F)
[Source: Concorde]
Last Updated on October 28, 2007
13.1. How Do I Prevent Permanent Sulfation?
13.2. So How Do I Store (Or Winterize) My Battery?
All lead-acid batteries are perishable. If not used weekly, people kill more deep cycle and power sport batteries with bad charging and maintenance practices, than batteries will die of old age!
When a lead-acid battery is discharged, soft lead sulfate crystals are formed in the pores and on the surfaces of the positive and negative plates. When left in a discharged condition or excessive high temperatures, is continually undercharged, or the electrolyte level is below the top of the plates or stratified, some of the soft lead sulfate re-crystallizes into hard lead sulfate. These crystals cannot be reconverted during subsequent recharging. This creation of hard crystals is commonly called permanent "sulfation". It is the leading cause and accounts for approximately 85% of the premature failures of lead-acid batteries not used on weekly basis. The longer sulfation occurs, the larger and harder the lead sulfate crystals become. The positive plates will turn a light brown and the negative plates will be dull, off-white. These permanent crystals lessen a battery's capacity and ability to be recharged or hold a charge. Sulfation primarily occurs in deep cycle and power sport batteries that are typically used for short periods and then are stored for long periods where they slowly self-discharge. Whereas a car or motorcycle starting battery is normally used several times a month, so permanent sulfation rarely becomes a problem unless it is unused or stored for long periods.
While a battery is in storage or not being used, the discharge is a result of parasitic load or natural self-discharge. Parasitic load is the constant electrical load present on a battery while it is installed in a vehicle even when the ignition key is turned off. The load is from the continuous operation of electrical appliances, such as an emissions computer, clock, security system, maintenance of radio station presets, etc. While disconnecting the negative battery cable will eliminate the parasitic load, it has no affect on the natural self-discharge of the battery. Thus, permanent sulfation can be a huge problem for lead-acid batteries while sitting for long periods on a dealer's shelf, in a basement, cellar, barn or garage, or in a parked vehicle, especially in hot temperatures.
13.1. How Do I Prevent Permanent Sulfation?
Please see Section 16.2 for more information on preventing sulfation.
13.2. So How Do I Store (Or Winterize) My Battery?
Batteries naturally self-discharge 1% to 60% per month (depending on the battery type and temperature) while not in use. Sulfation will begin occurring whenever the Depth-of-Discharge (DoD) increases above 0% in other words, when the battery is not fully charged. Please see Section 16 for more information on sulfation. Cold will slow the process down and heat will increase it up. Storing batteries under 250 AH on concrete floors will not normally cause them to naturally self-discharge faster. Please see Section 14.1 for more information on this myth. Below are six simple steps while your batteries are not in use to protect them from permanent sulfation and premature failure.
13.2.1. Physically inspect for leakage or damaged cases, remove any corrosion, clean and dry the tops of the batteries to remove possible discharge paths from dried battery electrolyte, and clean the terminals. If the battery is in a vehicle, remove the negative connection from the battery to eliminate the additional parasitic (key off) discharge.
13.2.2. If the battery has filler caps, check the electrolyte (battery acid) level in each cell. If required, add only distilled, deionized or demineralized water to the recommended level, but do not overfill.
13.2.3. Fully charge and equalize wet (flooded) batteries, if required, using the procedures in Section 9 and recheck the electrolyte levels when the battery cools.
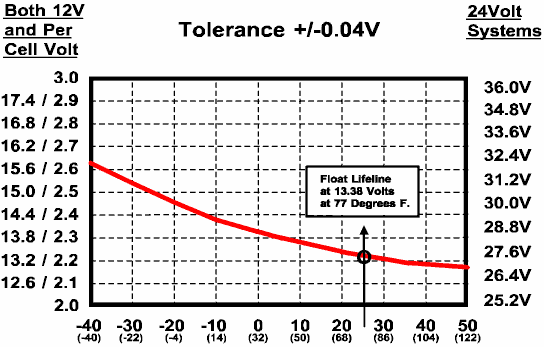
13.2.4. Store in a cold dry place, but not so that it will freeze, and where it can be easily recharged. The freezing point of a battery is determined by the SoC and the higher it is, the lower the freezing temperature. Please see the Electrolyte Freeze Points Table in Section 4.4.1. Based on the battery type you are using, connect a "smart", microprocessor based three-stage, four-stage charger or a voltage regulated float charger to continuously "float" charge your battery. Do not use a cheap, unregulated "trickle" charger or a manual two-stage charger which was not designed for "float" charging or you will overcharge your battery. A less desirable alternative to float charging would be to periodically test the State-of-Charge using the procedure in Section 4. When it is 80% or below, recharge using the procedures in Section 9. The frequency of testing and recharging will depend on the ambient storage temperature.
AGM (Ca/Ca) VRLA BATTERY FLOAT CHARGING VOLTAGE

TEMPERATURE IN DEGREES C (F)
[Source: Concorde]
13.2.5. Periodically test the State-of-Charge (SoC) and ensure that the electrolyte is at the proper levels.
13.2.6. Float or periodic recharging will prevent batteries from freezing. An Electrolyte Freeze Points at Various States-of-Charge for a Wet Lead-Acid Battery table indicates the temperature when the electrolyte will freeze.
13.2.7. When you remove the batteries from storage, charge and equalize, if required, using the battery manufacturer's recommended charging procedures or, if not available, the one in Section 9.